 |
The Left Hand of the Electron |
13 - THE EUREKA PHENOMENON
Scientists are wedded to reason, to the meticulous working
out of consequences from assumptions, to the careful organization of experiments
designed to check those consequences. If a certain line of experiments ends
nowhere, it is omitted from the final report. If an inspired guess turns
out to be correct, it is not reported as an inspired guess. Instead,
a solid line of voluntary thought is invented after the fact to lead up to
the thought, and that is what is inserted in the final report.
The result is that anyone reading scientific papers would swear that
nothing took place but voluntary thought maintaining a steady clumping
stride from origin to destination, and that just can't be true.
It's such a shame. Not only does it deprive science of much of its glamour
(how much of the dramatic story in Watson's Double
Helix do you suppose got into the final reports announcing the great
discovery of the structure of DNA ?*), but it hands
over the important process of 'insight',
'inspiration', 'revelation' to the
mystic.
* I'll tell you, in case you're curious. None!
The scientist actually becomes ashamed of having what we might call a revelation,
as though to have one is to betray reason - when actually what we call revelation
in a man who has devoted his life to reasoned thought, is after all merely
reasoned thought that is not under voluntary control.
Only once in a while in modern times do we ever get a glimpse into the workings
of involuntary reasoning, and when we do, it is always fascinating. Consider,
for instance, the case of Friedrich August Kekulé von Stradonitz.
In Kekulé's time, a century and a quarter ago, a subject of great
interest to chemists was the structure of organic molecules (those associated
with living tissue). Inorganic molecules were generally simple in the sense
that they were made up of few atoms. Water molecules,
for instance, are made up of two atoms of hydrogen and one of oxygen
(H20). Molecules of
ordinary salt
are made up of one atom of sodium and one of chlorine (NaCl), and so
on.
Organic molecules, on the other hand, often contained a large number of atoms.
Ethyl alcohol molecules have two carbon atoms, six hydrogen atoms, and an
oxygen atom (C2H60); the molecule of ordinary cane
sugar is C12H22011, and other molecules
are even more complex.
Then, too, it is sufficient, in the case of inorganic molecules generally,
merely to know the kinds and numbers of atoms in the molecule; in organic
molecules, more is necessary. Thus, dimethyl ether has the formula
C2H60, just as ethyl alcohol does, and yet the two
are quite different in properties. Apparently, the atoms are arranged differently
within the molecules - but how to determine the arrangements?
In 1852, an English chemist, Edward Frankland, had noticed that the atoms
of a particular element tended to combine with a fixed number of other atoms.
This combining number was called 'valence'. Kekulé in 1858 reduced
this notion to a system. The carbon atom, he
decided (on the basis of plenty of chemical evidence) had a valence of four;
the hydrogen atom, a valence of one; and the oxygen atom, a valence of two
(and so on).
Why not represent the atoms as their symbols plus a number of attached dashes,
that number being equal to the valence. Such atoms could then be put together
as though they were so many Tinker Toy units and 'structural formulas' could
be built up.
It was possible to reason out that the structural formula of ethyl alcohol
was
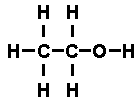
while that of di-methyl ether was
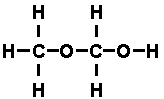
In each case, there were two carbon atoms, each with four dashes
attached; six hydrogen atoms, each with one dash attached; and an oxygen
atom with two dashes attached. The molecules were built up of the same
components, but in different arrangements.
Kekulé's theory worked beautifully. It has been immensely deepened
and elaborated since his day, but you can still find structures very much
like Kekulé's Tinker Toy formulas in any modern chemical textbook.
They represent oversimplifications of the true situation, but they remain
extremely useful in practice even so.
The Kekulé structures were applied to many organic molecules in the
years after 1858 and the similarities and contrasts in the structures neatly
matched similarities and contrasts in properties. The key to the rationalization
of organic chemistry had, it seemed, been found. Yet there was one disturbing
fact. The well-known chemical benzene wouldn't fit. It was known to have
a molecule made up of equal numbers of carbon and hydrogen atoms. Its molecular
weight was known to be 78 and a single carbon-hydrogen combination had a
weight of 13. Therefore, the benzene molecule had to contain six carbon-hydrogen
combinations and its formula had to be C6H6. But that
meant trouble. By the Kekulé formulas, the hydrocarbons (molecules
made up of carbon and hydrogen atoms only) could easily be envisioned as
chains of carbon atoms with hydrogen atoms attached. If all the valences
of the carbon atoms were filled with hydrogen atoms, as in 'hexane', whose
molecule looks like this-
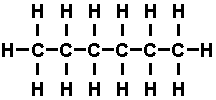
the compound is said to be saturated. Such saturated hydro carbons
were found to have very little tendency to react with other substances. If
some of the valences were not filled, unused bonds were added to those connecting
the carbon atoms. Double bonds were formed as in 'hexene'-
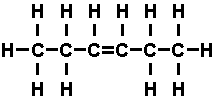
Hexene is unsaturated, for that double bond has a tendency to
open up and add other atoms. Hexene is chemically active. When six carbons
are present in a molecule, it takes fourteen hydrogen atoms to occupy all
the valence bonds and make it inert - as in hexane. In hexene, on the other
hand, there are only twelve hydrogens. If there were still fewer hydrogen
atoms, there would be more than one double bond; there might even be triple
bonds, and the compound would be still more active than hexene.
Yet benzene, which is C6H6 and has eight fewer hydrogen
atoms than hexane, is less active than hexene, which has only two
fewer hydrogen atoms than hexane. In fact, benzene is even less active than
hexane itself. The six hydrogen atoms in the benzene molecule seem to satisfy
the six carbon atoms to a greater extent than do the fourteen hydrogen atoms
in hexane.
For heaven's sake, why?
This might seem unimportant. The Kekulé formulas were so
beautifully suitable in the case of so many compounds
that one might simply dismiss benzene as an exception to the general rule.
Science, however, is not English grammar. You
can't just categorize something as an exception. If the exception doesn't
fit into the general system, then the general system must be wrong.
Or, take the more positive approach. An exception can often be made to
fit into a general system, provided the general system is broadened. Such
broadening generally represents a great advance and for this reason, exceptions
ought to be paid great attention.
For some seven years, Kekulé faced the problem of benzene and tried
to puzzle out how a chain of six carbon atoms could be completely satisfied
with as few as six hydrogen atoms in benzene and yet be left unsatisfied
with twelve hydrogen atoms in hexene.
Nothing came to him!
And then one day in 1865 (he tells the story himself) he was in Ghent, Belgium,
and in order to get to some destination, he boarded a public bus. He was
tired and, undoubtedly, the droning beat of the horses' hooves on the
cobblestones, lulled him. He fell into a comatose half-sleep.
In that sleep,
he seemed to see a vision of atoms attaching themselves to each other in
chains that moved about. (Why not? It was the sort of thing that constantly
occupied his waking thoughts.) But then one chain twisted in such a way that
head and tail joined, forming a ring - and Kekulé woke with a start.
To himself, he must surely have shouted 'Eureka', for indeed he had it. The
six carbon atoms of benzene formed a ring and not a chain, so that the structural
formula looked like this:
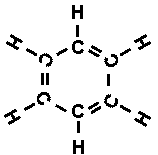
To be sure, there were still three double bonds, so you
might think the molecule had to be very active - but now there was a difference.
Atoms in a ring might be expected to
have different properties
from those in a chain and double bonds in one case might not have the
properties of those in the other. At least, chemists could work on that
assumption and see if it involved them in contradictions.
It didn't. The assumption worked excellently well. It turned out that organic
molecules could be divided into two groups: aromatic and aliphatic. The former
had the benzene ring (or certain other similar rings) as part of the structure
and the latter did not. Allowing for different properties within each group,
the Kekulé structures worked very well.
For nearly seventy years, Kekulé's vision held good in the hard field
of actual chemical techniques, guiding the chemist through the jungle of
reactions that led to the synthesis of more and more molecules. Then, in
1932, Linus Pauling applied quantum mechanics to
chemical structure with sufficient subtlety to explain just why the benzene
ring was so special and what had proven correct in practice proved correct
in theory as well.
Other cases? Certainly.
In 1764, the Scottish engineer James Watt was working as an instrument maker
for the University of Glasgow. The university
gave him a model of a Newcomen steam engine, which didn't work well, and
asked him to fix it. Watt fixed it without trouble, but even when it worked
perfectly, it didn't work well. It was far too inefficient and consumed
incredible quantities of fuel. Was there a way to improve that?
Thought didn't help; but a peaceful, relaxed walk on a Sunday afternoon did.
Watt returned with the key notion in mind of using two separate chambers,
one for steam only and one for cold water only, so that the same chamber
did not have to be constantly cooled and reheated to the infinite waste of
fuel.
The Irish mathematician
William Rowan
Hamilton worked up a theory of
'quaternions'
in 1843 but couldn't complete that theory until he grasped the fact that
there were conditions under which p xq was not
equal to q xp. The necessary thought came to him in a flash one time
when he walking to town with his wife. The German physiologist Otto Loewi
was working on the mechanism of nerve action, in particular, on the chemicals
produced by nerve endings. He woke at 3 A.M. one night in 1921 with a perfectly
clear notion of the type of experiment he would have to run to settle a key
point that was puzzling him. He wrote it down and went back to sleep. When
he woke in the morning, he found he couldn't remember what his inspiration
had been. He remembered he had written it down, but he couldn't read his
writing.
The next night, he woke again at 3 A.M. with the clear thought once more
in mind. This time, he didn't fool around. He got up, dressed himself, went
straight to the laboratory and began work. By 5 A.M. he had proved his point
and the consequences of his findings became important enough in later years
so that in 1936 he received a share in the Nobel prize in medicine and
physiology.
How very often this sort of thing must happen, and what a shame that scientists
are so devoted to their belief in conscious thought that they so consistently
obscure the actual methods by which they obtain their results.
 by NELSON JONES by NELSON JONES Ever wondered about the snake on the front cover of this magazine, coiled in a circle with its tail in its mouth? The snake swallowing its tail is called an ouroboros (Greek for tail-devourer),it's an ancient symbol of wholeness regeneration and the cycles of life which is found ,all over the world. In alchemy, the ouroboros represents the oneness of the universe. The head biting the tail forms an unbroken circle; having no sides or exits, the circle has always symbolised unity and totality. Often the ouroboros figure is accompanied by the Greek phrase 'Hen to pan,' which means 'All is one.' This points to the alchemists' belief that all the matter in the cosmos is composed of the same prime material: this was what made possible the transformation of base metal into gold, the purest substance. The ouro-boros also symbolised the 'prime matter' in the alchemist's laboratory, the stage at which the ingredients are waiting to be purified and transformed. More generally, it signified the potentiality of the universe. Sometimes the serpent is shown coiling around an egg. Here, the egg symbolises the prime matter while the ouroboros is wrapped around it protectively, like a dragon guarding treasure. This concept, originally developed by the mystical Greek sect of the Orphics (from whom Pythagoras drew inspiration) contains a mixture of symbolism. The idea of a protective circle is familiar in many contexts, from the practice of magic to old Westerns in which wagons fend off Indian raids by forming an impenetrable circle. In classical mythology, the world was encircled by the river Ocean. Snakes are symbolically associated with water, which in turn has often been seen as the source of life. In Mesopotamian myth, for example, the primordial waters were the domain of Tiamat, the dragon -goddess whose struggle with the god Marduk was dramatised in the epic of Creation. The scholar Mircea Eliade elaborated the concept of the Eternal Return: the idea that in myth and ritual we return to a sacred time, the point of origin celebrated in creation myths which is also outside space and time and thus eternally present. This is the Dream Time of the Australian Aborigines. The ouroboros is a metaphor for this perpetual quest; finding renewal by recreating the beginning of things. In the modern West we are taught to view time as linear, beginning in a Big Bang and ending, several billion years hence, possibly in a Big Crunch. But most cultures have seen time as moving in circles, endlessly returning, and the ouroboros embodies this idea on many levels. In Buddhism it is a symbol of the great cosmic wheel of Samsara. In Egyptian art, it represents the movement of the Sun across the heavens each day. On tombstones, the ouroboros is a symbol of immortality and rebirth. The ancients understood that life and death are not opposites but, rather, part of the same cycle. In nature, dead matter both plant and animal is the source of nutrients and new life: death is part of life and life depends on death. Looked at in one way the snake is devouring itself, absorbing its own essence, which seems futile. But look again: in fact a new snake is growing, tail first, from the head of the old. This is just one of the paradoxes of the ouroboros. Like the Oriental yin-yang symbol, it exemplifies the union of opposites, the dynamic balance between polarities such as life and death, male and female, stasis and change. The ouroboros reminds us that the universe is a self-sustaining whole. The serpent takes from itself, grows and returns to its point of origin where the cycle begins afresh. It is a diagram of the endless process of absorption and emission that sustains the natural cycles of life - like a river constantly flowing back to its own source. This isn't merely a symbolic message. These days, when we have learned that the planet resources are finite, the ouroboros might be a mode for sustainable living - a dramatic illustration of recycling! It is also a symbol of the chthonic energies which flow through the Earth, and of the energy that flows through the human body, which in Indian philosophy is known as kundalini or serpent power and resides at the base of the spine. Every electrician knows that power has to be earthed and mystical philosophy stresses that the body's energies need to be allowed to flow freely. With the ouroboros, the head biting the tail completes the circuit, permitting the vivifying current to pass. It's a symbol, too, of wisdom endlessly renewing itself. The snake represents the wisdom of the Earth. The coiled snake is the circle of knowledge in which everything is linked. It demonstrates the ancient belief that knowledge is a unified, indivisible whole - in contrast to the modern tendency for ever narrower fields of specialisation. The fact that the ouroboros is found throughout the world suggests that it is one of those signs (in Jungian terms, an archetype) that reside in the unconscious and through which concepts are mediated. As an example, take the 19th century German chemist August Kekule who was trying to discover the structure of the benzene molecule (more important than it sounds!). He got nowhere until one night he dreamed of ouroboros. At once he had the answer: the molecule formed itself into a ring. The ouroboros taps into up ancient roots yet always something new to say. Just like PREDICTION, in fact! [Predictions magazine July 1997 p26] |
